What are the FDA Labeling Requirements for Lip Balm Labels?

Have you ever wondered how lip balm companies create their labels?
Lip balm labels do more than just show a beautiful design; they also help inform customers of important information. Essential features like ingredient lists and safety warnings are provided to promote transparency, safety and ensure that customers are fully aware of what's inside a product. If you're a small business owner or simply looking to understand lip balm labeling requirements, this guide will walk you through the basic requirements and help you label your products correctly.
How Does the Food and Drug Administration (FDA) Classify Lip Balms?
Before we start, it's essential to understand how the Food and Drug Administration (FDA) defines cosmetics. According to the FDA, a cosmetic is a product whose primary purpose is to be applied to the human body to clean or beautify an individual. Lip balms as cosmetics can be divided in two main groups:
Cosmetic Lip Balms (Moisturizing Purposes Only)
These products are designed exclusively to moisturize and improve the look and feel of lips without making any medical claims or including any additional medicine or ingredients. Common ingredients in lip balms include beeswax, shea butter, coconut oil, etc., all known for providing a smooth and hydrating experience.

OTC Drug Lip Balms (Sun Protection or Medicated Benefits)
Drug Lip balm products on the other hand claim to treat, heal, or prevent any condition, and oftenly have ingredients such as menthol, camphor, hydrocortisone, etc. To differentiate them from Cosmetic Lip Balms, add vocabulary like "medicated” or "hypoallergenic," which must meet specific FDA requirements, as well as the active ingredients list and proper material uses.

Since both product types aim to clean, beautify, or improve health conditions, they are considered drugs according to the FDA's criteria. Section 509 of the FD&C Act explains that the categories of "drug" and "cosmetic" are not exclusive, and a lip balm can be named with both names. It must also follow cosmetic and drug labeling requirements, including essential ingredients, labeling rules, and a Drug Facts panel.
How to Place Information on a Lip Balm Label
The information on a product is usually showcased in the label and contains information such as name, price, ingredients, among others. According to Section 602c of the FDA, a product can be taken off the market if the information is not placed correctly or written in a way the client understands. Therefore, here is a visual representation of how a lip balm should showcase its information according to the FDA:
Information on the Outer Container:
Front:
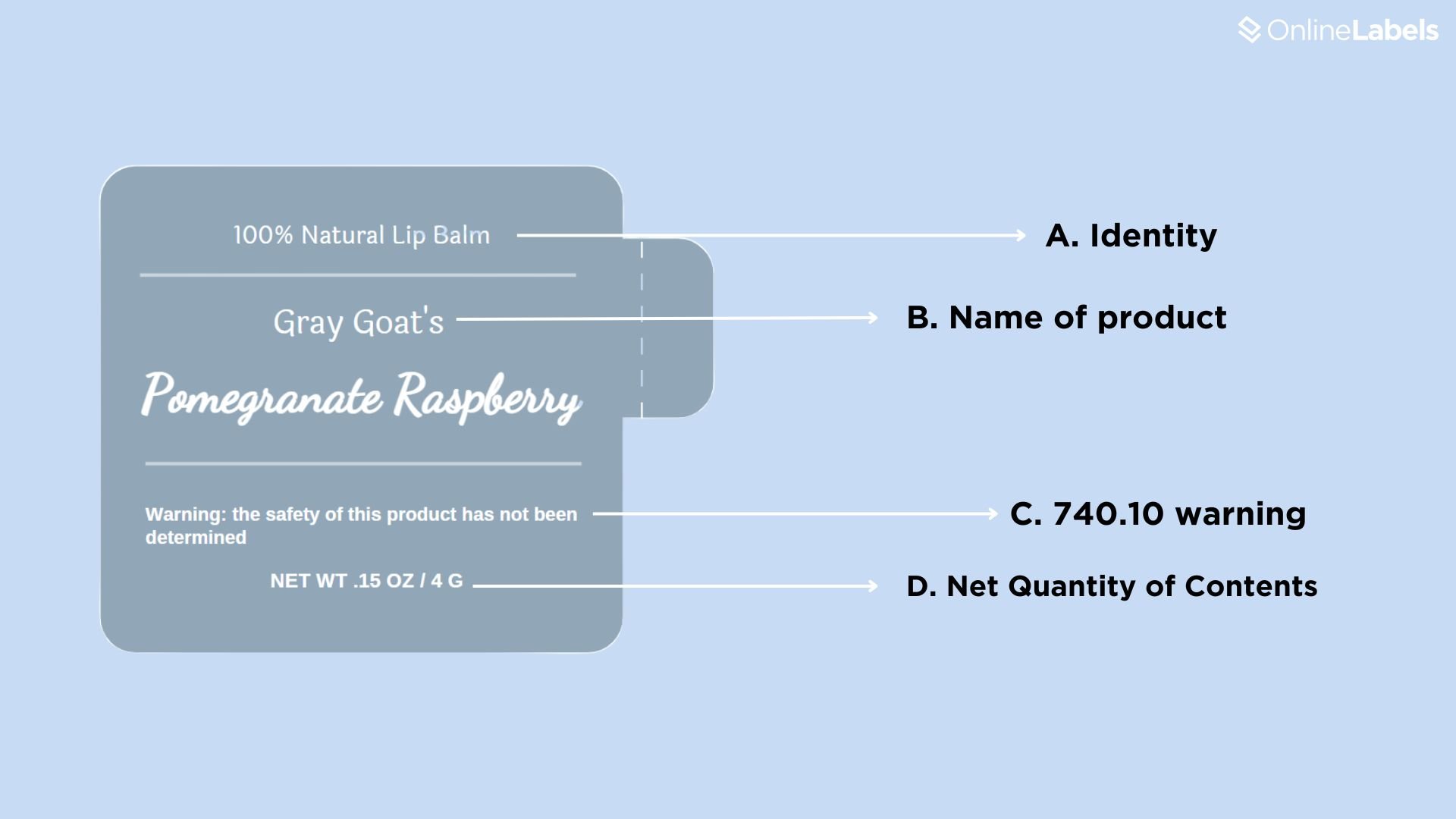
- A. Identity: the product type, which can vary depending on the product, such as organic lip balm.
- B. Name of product: this should include the company's legal and patented name.
- C. 740.10 warning: this is only added if the product contains an ingredient from which appropriate proof of safety hasn't been obtained.
- D. Net quantity of contents: the quantity of the contents in terms of weight.
Back:
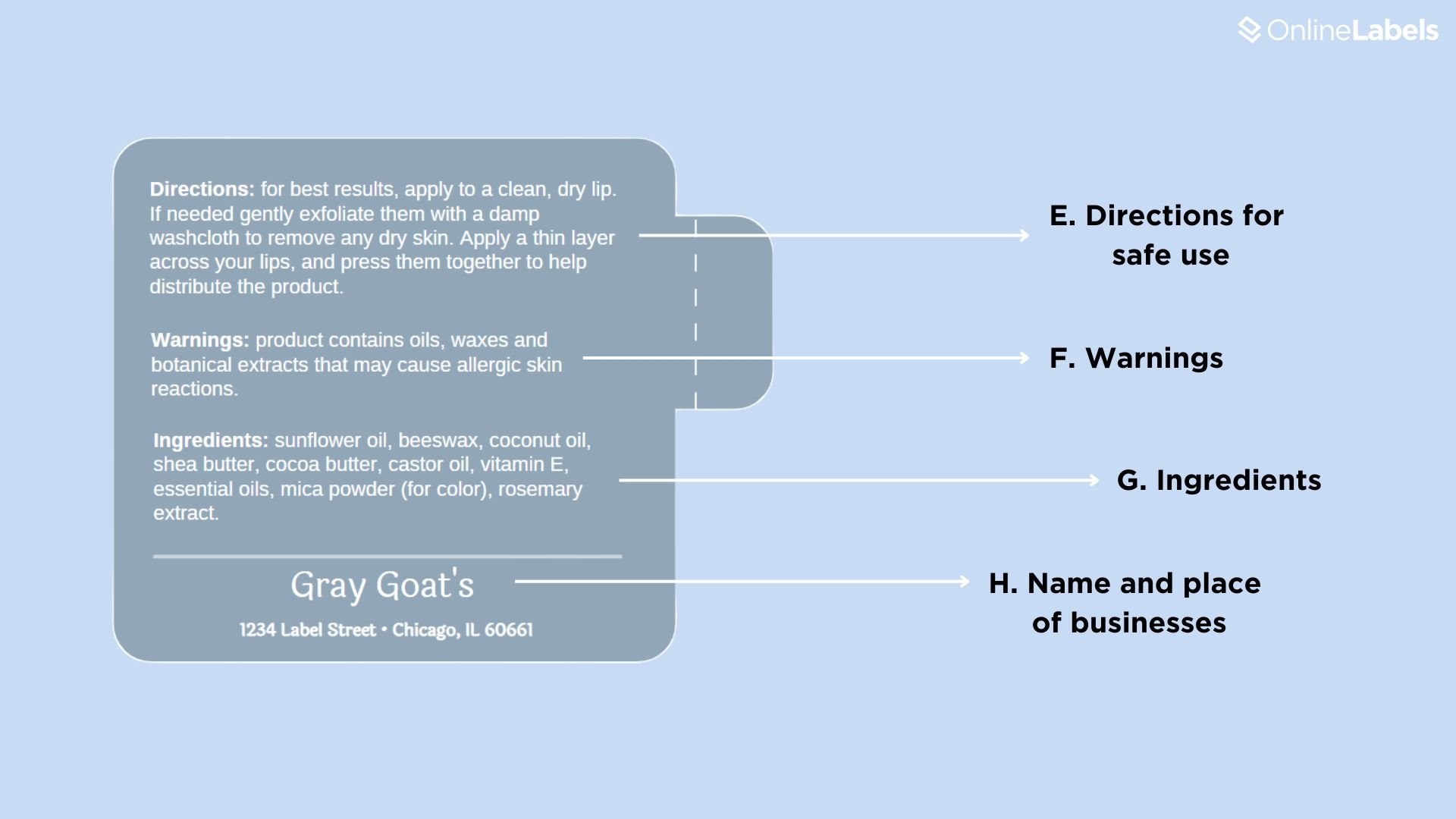
- E. Directions for safe use: this contains clear instructions on how to use the product.
- F. Warnings: includes potential risks of the product, including allergies.
- G. Ingredients: list all components at their respective weight levels; if other sub-ingredients form an ingredient, they must be placed on the bottle. For example, if your lip balm includes Optiphen Plus (comprised of Phenoxyethanol, Caprylyl Glycol, and Sorbic Acid), all sub-ingredients must also be listed.
- H. Name and place of businesses: this includes the address and place where the products have been produced.
How to Adjust a Lip Balm Label to Meet FDA Design Requirements
When designing a lip balm label, it must comply with FDA regulations, having all fonts legible and information prominently being placed on the label. Some of the main factors that the FDA requires include:
- Language
- Type Size
- Net Quantity Statements
Language
Since the FDA follows regimes from the United States, the primary language required is English. However, according to Section 701.2 (b) there can be exceptions:
- English language statements: all elements included in a lip balm label must be in English. Products produced and sold in US territories like Puerto Rico or Guam should also follow this rule.
- Foreign language statements: if the label is in another international language, the required legal elements must appear in English.
Type Size
For letter sizing and general ingredient information, the letters must be less than 1/16" in height according to Section 701.3 (b); however, if the space available is less than 12 square feet, the letter sizing must be 1/32". For Warning Statements, the size should always be 1/16” unless a smaller size is established.
Net Quantity Statements
Some of the main items to take into consideration when adding Net Quantity Statements to your labels include:
- Accuracy: the net amount of cosmetics included in the product; this can consist of weight, volume, etc.
- Product consistency: the product must be included in terms of liquid at 68°F (20°C).
- Systems: this is the weight and should be included in pounds and ounces.
- Unit terms: "Net Weight" or “net wt." must be used.
These are a few examples of FDA-approved Net Quantity Statements:
- Net wt. 5 av. oz.
- Net contents 5 fl. oz.
- 5 av. oz. net wt.
- 5 oz. net wt.
- Net 5 fl. oz.
- 5 fl. Oz.
If the product is less than 4 pounds, it must reveal the total number of ounces, similar to the following examples:
- Net Wt. 25 oz. (1 lb. 8 oz.)
- Net Wt. 25 oz. (1 - 1/2 lb.)
- Net Wt. 25 oz. (1.5 lb.)
- 57 fl. oz. (1 qt. 1 pt. 8 fl. oz.)
Cosmetic Warning Statements
Regulations for lip balm labels must be placed on the back of the product. This should include if the product presents any health hazard or allergy trigger. A cosmetic that doesn't include a warning statement can be considered misbranded, according to Section 602 (a).
The letter type for this section should be 1/16 inches and have a color that helps contrast the background to make it legible for the reader.
How to Design a Lip Balm Label in Maestro Label Designer
Now that you have the basics of creating a lip balm label that follows FDA guidelines, you can start designing your labels with our user-friendly software, Maestro Label Designer. Here’s an easy step-by-step guide to get started.
1. Launch Maestro Label Designer.
2. Once you log in, select Pre-Designed Templates on the upper left side and browse for Lip Balm Labels.
3. Choose the label design that you prefer the most.
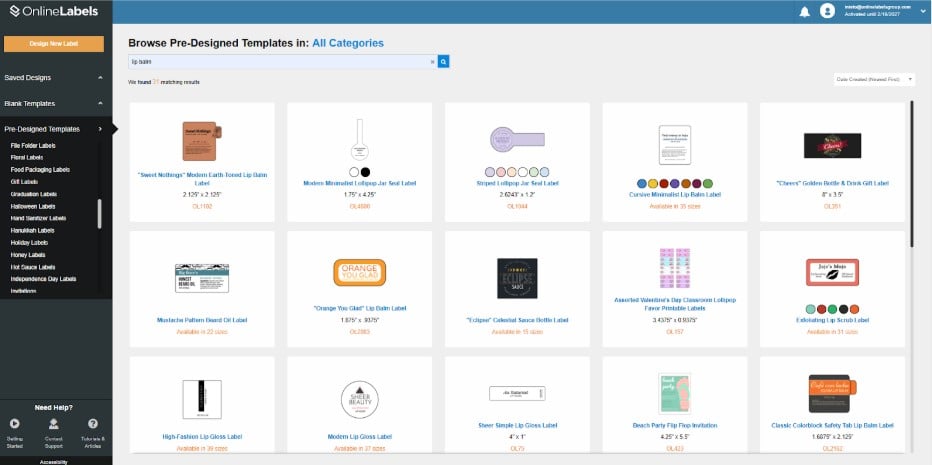
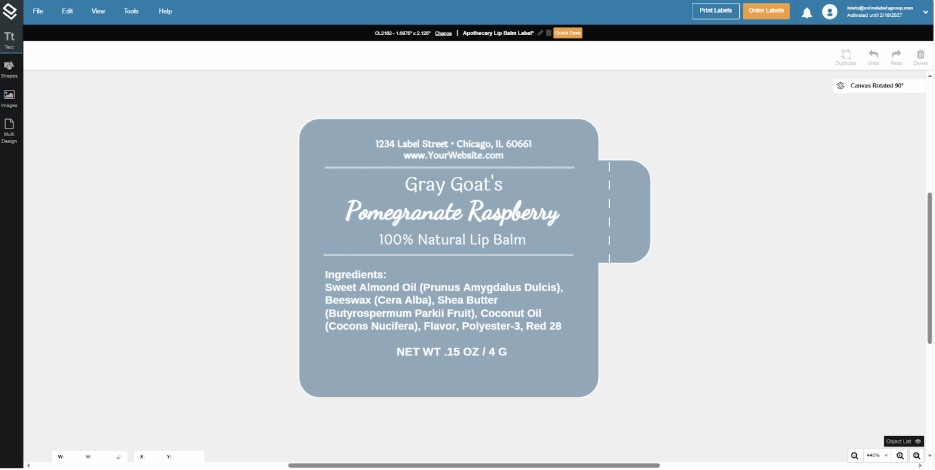
4. When you choose your label, you can start designing it with the tools in the upper left corner of your screen.
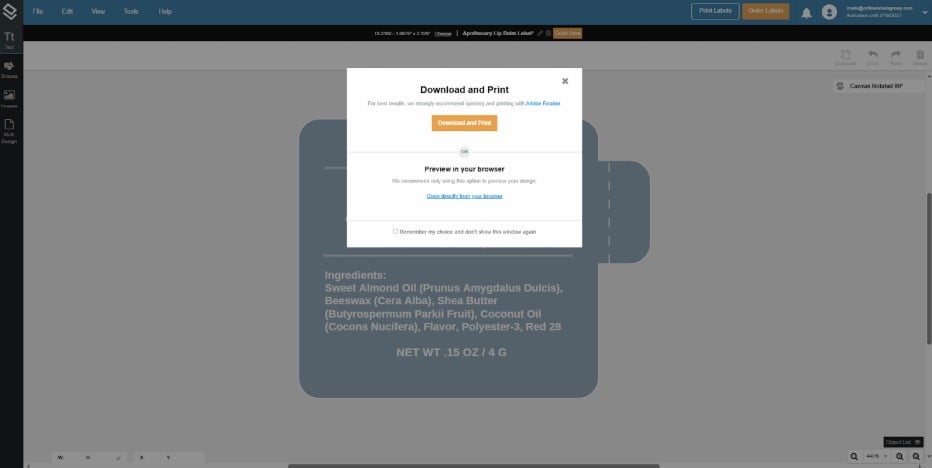
5. Once it's ready, you can print the labels yourself by clicking on the upper right corner and selecting the Print Labels button.
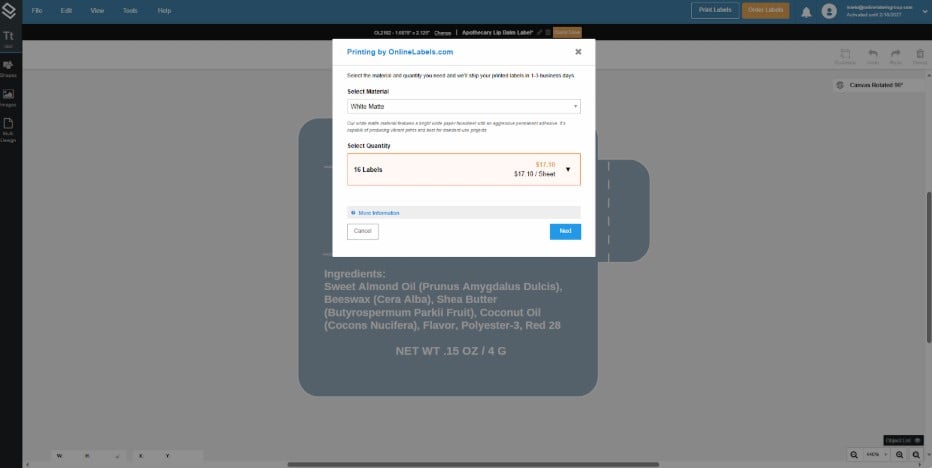
6. If you wish to order custom labels from us, click on the Order Labels button, which is also in the upper right corner of your screen. You can select the quantity and material of your order, and we'll handle the rest.
Lip Balm Labels: Easy and Simple
Creating a compliant and well-designed lip balm label is essential for legal requirements and customer trust. By knowing FDA norms and regulations, labeling requirements, and design guidelines, you can ensure that your product meets industry standards while maintaining a professional and eye-catching design. Please visit the FDA website for more specific information on warning statements, net quantity of contents declaration, and more!


